The Deceptive Simplicity of a Chemical Formula
The seemingly simple combination of an organic molecule and water, represented by the formula hcooch ch2 h2o, belies a world of chemical complexity and profound industrial significance. This formula is a condensed representation of a fundamental reaction in organic chemistry: the hydrolysis of an ester. To the uninitiated, it is a string of atomic symbols; to the chemist,
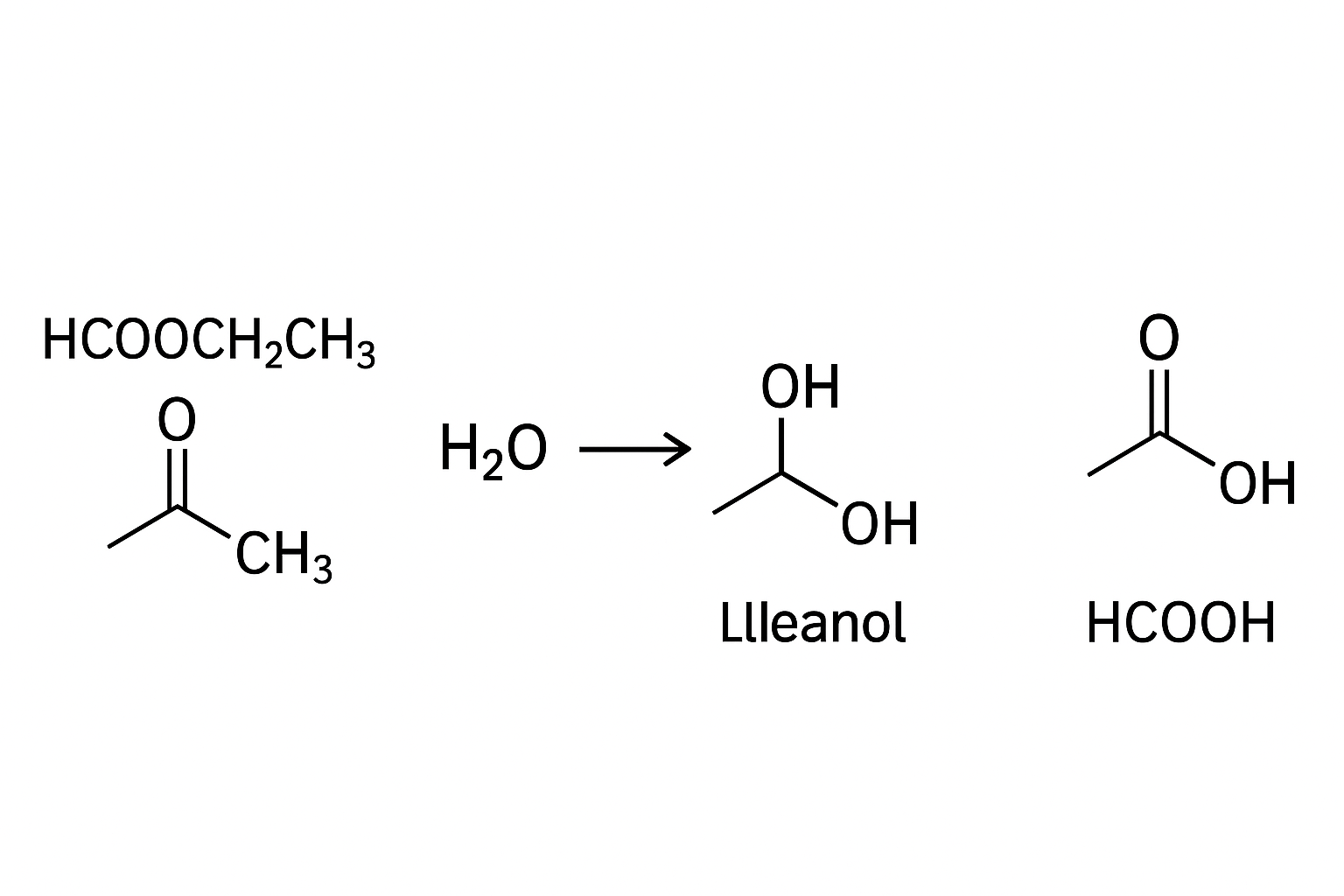
it is an equation waiting for its products, a story of bond-breaking and bond-making, and a process vital to industries from food to fuel. The specific compound HCOOCH₂CH₃ is known as ethyl methanoate, or more commonly, ethyl formate. Understanding what occurs when it encounters water is to understand a cornerstone reaction that shapes materials, flavors, and even life itself.
Deconstructing the Reactants: Ethyl Methanoate and Water
To fully appreciate the products of the reaction between hcooch ch2 h2o, one must first deconstruct the reactants. The entity “hcooch ch2” is a skeletal form of ethyl methanoate. Breaking it down, “HCOO” is the formate or methanoate group, derived from formic acid (HCOOH), the simplest carboxylic acid. The “CH₂CH₃” is an ethyl group, derived from ethanol (CH₃CH₂OH). An ester is essentially the product of a reaction between a carboxylic acid and an alcohol, and hydrolysis is the reverse of this process.
Therefore, the reaction encapsulated by hcooch ch2 h2o is nature’s way of disassembling this construct, of returning the ester to its parent constituents. Water, the universal solvent and a key player in countless biochemical pathways, is the reagent that facilitates this molecular disassembly.
The Critical Role of Reaction Conditions
The question of products, however, is not as straightforward as it might seem. The outcome of the interaction between hcooch ch2 h2o is critically dependent on the conditions under which the reaction takes place. There are two primary pathways, each with distinct mechanisms, catalysts, and practical implications. The first is acid-catalyzed hydrolysis, and the second is base-promoted hydrolysis, also known as saponification. The journey of the hcooch ch2 h2o system differs dramatically depending on the pH of its environment.
Pathway One: Acid-Catalyzed Hydrolysis and its Equilibrium
Let us first explore the acid-catalyzed hydrolysis pathway. In this scenario, the mixture of ethyl methanolate and water is treated with a strong mineral acid, typically sulfuric acid (H₂SO₄) or hydrochloric acid (HCl). The role of the acid catalyst is profound. It protonates the carbonyl oxygen atom of the ester, making the carbon atom of the carbonyl group significantly more electrophilic, or electron-loving.
This activated carbonyl carbon then becomes a prime target for nucleophilic attack by a water molecule. The mechanism proceeds through a series of proton transfers and the formation of a tetrahedral intermediate—a transient state where the carbonyl carbon becomes sp³ hybridized. This intermediate eventually collapses, leading to the expulsion of the alcohol component and the regeneration of the carboxylic acid. For the system defined by hcooch ch2 h2o, the specific products are methanoic acid (formic acid, HCOOH) and ethanol (CH₃CH₂OH). This reaction is an equilibrium process, meaning it can proceed in both directions.
Pathway Two: Base-Promoted Hydrolysis and its Irreversibility
The second, and in many ways more decisive, pathway is the base-promoted hydrolysis, or saponification. This is the fate of hcooch ch2 h2o in the presence of a strong base like sodium hydroxide (NaOH) or potassium hydroxide (KOH). Here, the hydroxide ion (OH⁻) from the base acts as a powerful nucleophile, directly attacking the electrophilic carbonyl carbon of the ester.
This, again, forms a tetrahedral intermediate. The critical difference from the acid-catalyzed route is what happens next. This intermediate collapses not by expelling an alkoxide, but by ejecting the much more stable alkoxide ion (CH₃CH₂O⁻). The alkoxide is a strong base and immediately deprotonates the carboxylic acid product. For ethyl methanoate, this means the initial organic product is the formate ion (HCOO⁻). The alcohol produced is ethanol.
The Historical Application: Saponification and Soap-Making
The transformation of hcooch ch2 h2o into its constituent parts is not merely a academic exercise confined to a laboratory flask. It has profound and widespread applications across numerous fields. The most historically significant application of ester hydrolysis, of which the reaction of hcooch ch2 h2o is a specific example, is soap-making.
Saponification, the name for base-promoted hydrolysis, is derived from the Latin word for soap, ‘sapo’. While traditional soaps are made from the hydrolysis of long-chain fatty acid esters (triglycerides) from fats and oils, the chemical principle is identical to that governing the breakdown of ethyl methanolate. The carboxylate salts produced are the soaps themselves, molecules with hydrophilic heads (the carboxylate group) and hydrophobic tails (the hydrocarbon chain) that emulsify grease and dirt.
Implications in Flavor, Fragrance, and Polymer Science
In the flavor and fragrance industry, the hydrolysis of esters is a double-edged sword. Many of the characteristic aromas of fruits—bananas, rum, strawberries—are due to the presence of specific esters. Ethyl methanolate itself has a characteristic rum-like smell. The controlled synthesis of these esters is crucial for creating artificial flavors. However, the inadvertent hydrolysis of these esters, perhaps due to moisture or microbial activity,
can lead to the loss of these desirable flavors and the appearance of the often less-pleasantly smelling carboxylic acids. The spoilage of wine, for instance, can involve hydrolysis and oxidation processes that alter its ester profile, leading to off-flavors. Thus, understanding and controlling the reaction symbolized by hcooch ch2 h2o is essential for both creating and preserving these sensory experiences.
The Biological Essentiality of Ester Hydrolysis
In biological systems, ester hydrolysis is a ubiquitous and life-sustaining process. Metabolic pathways are replete with reactions where enzymes, known as esterase’s, catalyze the hydrolysis of ester bonds with remarkable speed and specificity. The digestion of dietary fats is a prime example. Lipases in the gut catalyze the saponification of triglycerides, breaking them down into fatty acid salts (soaps, in a biological context) and glycerol, which are then absorbed and used for energy.
The entire reaction, while immensely more complex due to enzymatic control, is fundamentally the same hydrolysis reaction. Even the action of some neurotransmitters and the metabolism of many drugs involve the cleavage of ester linkages. The body’s ability to perform the transformation encapsulated by hcooch ch2 h2o is a fundamental tool of physiology and pharmacology.
Conclusion: A Formula of Profound Significance
In conclusion, the deceptively simple notation hcooch ch2 h2o serves as a gateway to a rich and multifaceted chemical narrative. It is the symbolic representation for the hydrolysis of ethyl methanoate. The products of this reaction are not a single, fixed pair, but are determined by the reaction medium. Under acidic conditions, the system reaches an equilibrium yielding methanoic acid and ethanol. Under the forceful drive of a strong base, the reaction proceeds irreversibly to completion, producing a carboxylate salt, such as sodium formate, and ethanol.
The significance of this transformation extends far beyond the balanced equation. It is the chemical heart of soap-making, a critical factor in flavor chemistry, a principle behind polymer recycling and degradation, and an essential mechanism in biological metabolism. The seventh and final use of the keyword hcooch ch2 h2o thus brings us full circle, reminding us that this concise formula is a powerful abstraction for a reaction that is both a fundamental tenet of organic chemistry and a vital process that resonates through our industries, our environment, and our very bodies. Understanding its products is to understand a fundamental language of molecular change.